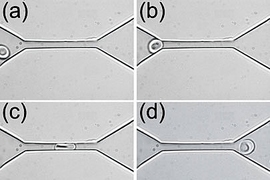
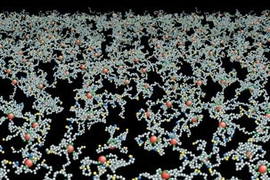
Millions of times during their four-month lifespan, human red blood cells must squeeze through tiny capillaries to deliver their payload of oxygen and pick up waste carbon dioxide-functions essential to life.
Now, for the first time, MIT researchers have developed a dynamic, molecular-level model that describes how the cells deform their normal disc shape to pass through vessels that are often much narrower than the cells themselves.
Blood cells must rearrange components of their internal scaffolding (so-called cytoskeleton), allowing the cells to become almost liquid-like, in order to squeeze through the narrowest capillaries found in the body, the researchers report in a paper to be published in the March 12 online edition of the Proceedings of the National Academy of Sciences.
Studying the mechanics of how a blood cell can transform from a soft object to an almost fluid-like state will help researchers better understand several types of blood disorders, said Subra Suresh, senior author of the paper and the Ford Professor of Engineering with joint appointments in materials science and engineering, biological engineering, mechanical engineering and health sciences and technology.
"Now we can study how molecular structure affects the shape, which affects the mechanical properties, and both of which affect mobility," he said.
Mobility is a key factor in diseases like malaria and the genetic disorder sickle cell anemia, both of which render red blood cells unable to flow through narrow capillaries.
Red blood cells have a diameter of about eight microns, or millionths of a meter. As they flow through the body, they often encounter blood vessels, such as those in the brain, with a diameter of only about two microns. Each time the cells reach such a vessel, they must stretch into a bullet-like shape to squeeze through and then return to their original disc shape upon exiting the vessel.
The researchers' model shows that reorganization of the cytoskeleton could account for such deformation. Every red blood cell has a cytoskeleton, a sort of scaffolding made of protein molecules called spectrin, attached to the inside of its cell membrane in a brush-like network.
When the bonds within that protein network or between the network and the cell membrane are broken, holes open up in the cytoskeleton, allowing the cell to become more fluidic and squeeze through narrow passages. The researchers show that such a transformation can be achieved by breaking either of two types of cytoskeletal bonds-bonds between two molecules of spectrin or bonds between spectrin and another protein called actin, which is embedded in the cell membrane.
An input of either mechanical energy (such as squeezing or shearing) or chemical energy (such as ATP, an energy carrier used by cells), is enough to break those bonds and cause the necessary cytoskeleton deformation, according to Suresh. In the future, the researchers plan to study how the interplay of those types of energy inputs affects the cells.
The new model could also be used to study several types of blood disorders, including malaria, as the cell membrane and cytoskeleton are altered by the presence of the parasite inside the cell.
In earlier work, Suresh and colleagues showed that as malaria infection progresses, red blood cells become less deformable, which explains why it is harder for them to squeeze through narrow vessels. Using the new model, researchers can study how the infection affects the blood cells on a molecular level to make them less deformable.
Other diseases that could be studied are the genetic disorders sickle cell anemia and spherocytosis. In patients with sickle cell anemia, red blood cells take on a sickle shape that prevents them from flowing through blood vessels. Spherocytosis causes red blood cells to become spherical so they can't deform properly to get through small capillaries.
The lead author on the PNAS paper is Ju Li, a former MIT graduate student and assistant professor at Ohio State University. Other authors are George Lykotrafitis, a postdoctoral associate in MIT's Department of Materials Science and Engineering (MSE), and Ming Dao, an MIT research scientist in MSE.
The research was funded by the National Institutes of Health.
A version of this article appeared in MIT Tech Talk on March 14, 2007 (download PDF).