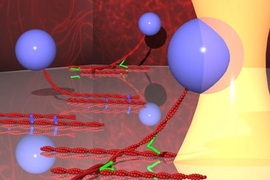
MIT researchers have developed a novel technique to measure the strength of the bonds between two protein molecules important in cell machinery: Gently tugging them apart with light beams.
"It's really giving us a molecular-level picture of what's going on," said Matthew Lang, an assistant professor of biological and mechanical engineering and senior author of a paper on the work appearing in the June 30 advanced online issue of the Proceedings of the National Academy of Science.
Last fall, Lang and others demonstrated that light beams could be used to pick up and move individual cells around the surface of a microchip.
Now they have applied the optical tweezers to measuring protein microarchitectures, allowing them to study the forces that give cells their structure and the ability to move.
The researchers focused on proteins that bind to actin filaments, an important component of the cytoskeleton. Depending on the arrangement and interaction of actin filaments, they can provide structural support, help the cell crawl across a surface or sustain a load (in muscle cells).
"We're trying to understand how nature engineered these molecular linkages to use in different ways," said Lang.
Actin filaments are most commonly found either bonded or crosslinked by a much smaller actin binding protein.
The researchers studied the interactions between the proteins by pinning one actin filament to a surface and controlling the motion of the second one with a beam of light. As the researchers tug on a bead attached to the second filament, the bond mediated by the actin-binding protein eventually breaks.
With this technique, the researchers can get a precise measurement of the force holding the proteins together, which is on the order of piconewtons (10^-12 newtons).
The same technique could be used to investigate many of the other hundreds of protein interactions that occur in the cytoskeleton, said Lang.
Lead author of the paper is Jorge Ferrer, a recent PhD recipient in biological engineering. Other MIT authors of the paper are Hyungsuk Lee and Benjamin Pelz, graduate students in mechanical engineering; and Roger Kamm, the Germeshausen Professor of Mechanical Engineering and Biological Engineering.
Jiong Chen of Stony Brook University and Fumihiko Nakamura of Harvard Medical School are also authors of the paper.
The research was funded by the Nicholas Hobson Wheeles Jr. Fellowship, the W.M. Keck Foundation, and the Westaway Research Fund.