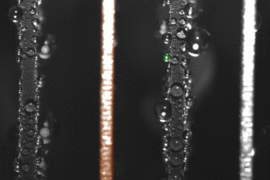Last year, MIT researchers discovered that when water droplets spontaneously jump away from superhydrophobic surfaces during condensation, they can gain electric charge in the process. Now, the same team has demonstrated that this process can generate small amounts of electricity that might be used to power electronic devices.
The new findings, by postdoc Nenad Miljkovic, associate professor of mechanical engineering Evelyn Wang, and two others, are published in the journal Applied Physics Letters.
This approach could lead to devices to charge cellphones or other electronics using just the humidity in the air. As a side benefit, the system could also produce clean water.
The device itself could be simple, Miljkovic says, consisting of a series of interleaved flat metal plates. Although his initial tests involved copper plates, he says any conductive metal would do, including cheaper aluminum.
In initial testing, the amount of power produced was vanishingly small — just 15 picowatts, or trillionths of a watt, per square centimeter of metal plate. But Miljkovic says the process could easily be tuned to achieve at least 1 microwatt, or millionth of a watt, per square centimeter. Such output would be comparable to that of other systems that have been proposed for harvesting waste heat, vibrations, or other sources of ambient energy, and represents an amount that could be sufficient to provide useful power for electronic devices in some remote locations.
For example, Miljkovic has calculated that at 1 microwatt per square centimeter, a cube measuring about 50 centimeters on a side — about the size of a typical camping cooler — could be sufficient to fully charge a cellphone in about 12 hours. While that may seem slow, he says, people in remote areas may have few alternatives.
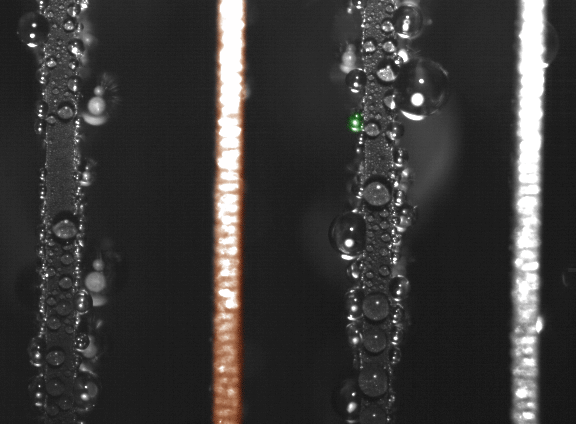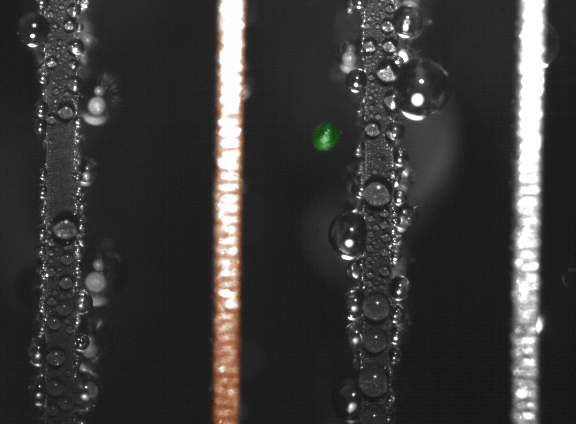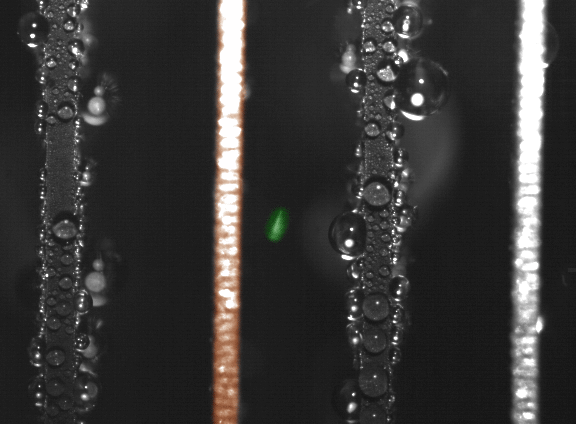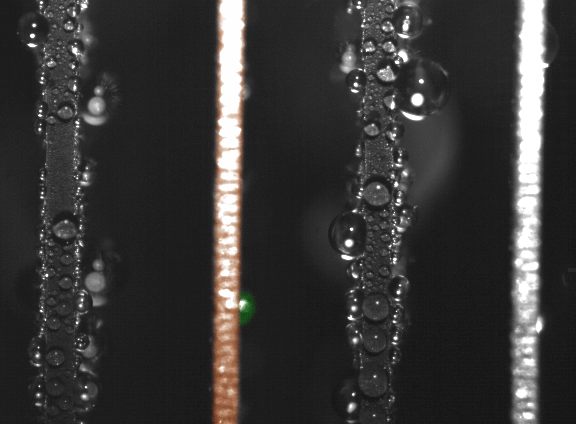
False-color time-lapse images captured via high-speed imaging show a droplet jumping (colored green) from a superhydrophobic copper oxide fin to a hydrophilic (water-attracting) copper fin (colored orange). (Courtesy of Nenad Miljkovic and Daniel J. Preston)
There are some constraints: Because the process relies on condensation, it requires a humid environment, as well as a source of temperatures colder than the surrounding air, such as a cave or river.
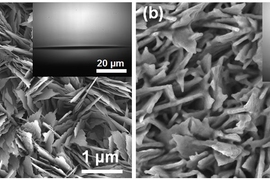
The system is based on Miljkovic and Wang’s 2013 finding — in attempting to develop an improved heat-transfer surface to be used as a condenser in applications such as power plants — that droplets on a superhydrophobic surface convert surface energy to kinetic energy as they merge to form larger droplets. This sometimes causes the droplets to spontaneously jump away, enhancing heat transfer by 30 percent relative to other techniques. They later found that in that process, the jumping droplets gain a small electric charge — meaning that the jumping, and the accompanying transfer of heat, could be enhanced by a nearby metal plate whose opposite charge is attractive to the droplets.
Now the researchers have shown that the same process can be used to generate power, simply by giving the second plate a hydrophilic surface. As the droplets jump, they carry charge from one plate to the other; if the two plates are connected through an external circuit, that charge difference can be harnessed to provide power.
In a practical device, two arrays of metal plates, like fins on a radiator, would be interleaved, so that they are very close but not touching. The system would operate passively, with no moving parts.
For powering remote, automated environmental sensors, even a tiny amount of energy might be sufficient; any location where dew forms would be capable of producing power for a few hours in the morning, Miljkovic says. “Water will condense out from the atmosphere, it happens naturally,” he says.
“The atmosphere is a huge source of power, and all you need is a temperature difference between the air and the device,” he adds — allowing the device to produce condensation, just as water condenses from warm, humid air on the outside of a cold glass.
Chuanhua Duan, an assistant professor of mechanical engineering at Boston University who was not involved in this research, says, “This work provides a new approach for energy-harvesting, which can be used to power [microelectromechanical] devices and small electronic devices.” He adds, “Getting power from a condensation process is definitely a novel idea, as condensation is mainly used for thermal management. … Recent studies of condensation on superhydrophobic surfaces [have] extended its applications in self-cleaning and anti-icing, but no one has correlated condensation with energy-harvesting before.”
The research, which also included MIT graduate student Daniel Preston and former postdoc Ryan Enright, now at Lucent Ireland Ltd., was supported by MIT’s Solid-State Solar-Thermal Energy Conversion Center (S3TEC), funded by the U.S. Department of Energy; the Office of Naval Research; and the National Science Foundation.